Intacs® Corneal Implants
The Only FDA Approved Intracorneal Segments
Intacs® Corneal Implants
The Only FDA Approved Intracorneal Segments
The Only FDA Approved Intracorneal Segments
The Only FDA Approved Intracorneal Segments
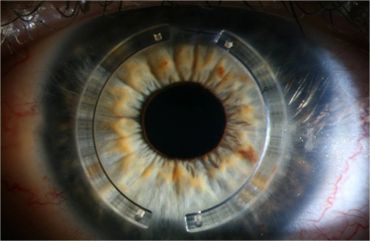
Intacs® Corneal Implants are ophthalmic medical devices designed for the reduction or elimination of myopia and astigmatism in patients with keratoconus so that their functional vision may be restored and the need for a corneal transplant procedure can potentially be deferred. When placed in the corneal stroma, outside of the patient’s central optical zone, the product reshapes and stabilizes the cornea.
Intacs® segments are designed to be placed in the periphery of the cornea, at approximately two-thirds depth, and are surgically inserted through a small radial incision in the corneal stroma.
Intacs® Corneal Implants are manufactured from polymethylmethacrylate (PMMA) and are available in different thicknesses. The Intacs® are designed to allow removal or replacement.
Keratoconus is a disease that creates a thinning of the cornea, causing it to progressively bulge into a cone-like shape. The change in the cornea's shape can have a dramatic impact on one's vision.
Intacs® may provide an effective option to improve one's vision prior to considering a cornea transplant.



We use cookies to analyze website traffic and optimize your website experience. By accepting our use of cookies, your data will be aggregated with all other user data.